
Carbon tetrachloride (C{Cl}_{4}):Ionic substanceNon-polar covalent substancePolar covalent substanceMacromolecular substanceMetallic substance
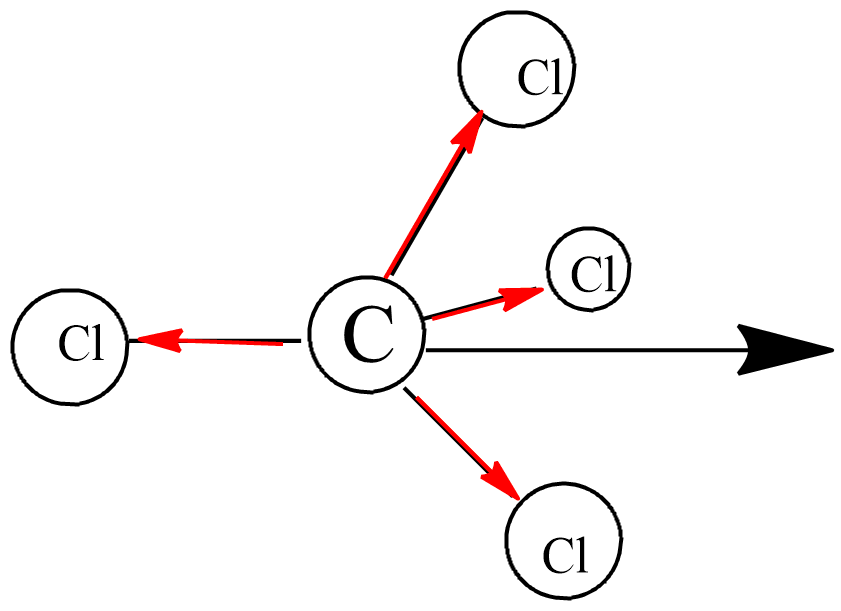
Carbon tetrachloride ($CC{{l}_{4}}$ ):(A) ionic substance(B) non-polar covalent substance(C) polar covalent substance (D) macromolecular substance(E) metallic substance

Polarity of Molecules 11/18/14 Polar Molecules are molecules which have an uneven distribution of charge. One side of the molecule is negative while. - ppt download

SOLVED: Explain how a molecule that contains polar bonds can be nonpolar. In your answer, use carbon tetrachloride, CCl4, as an example.

SOLVED: The four bonds of carbon tetrachloride (CCl4) are polar, but the molecule is nonpolar because the bond polarity is canceled by the symmetric tetrahedral shape. When other atoms substitute for some

Explain why carbon tetrachloride molecules are not polar while carbon trichloride is polar - Digital Teachers Uganda
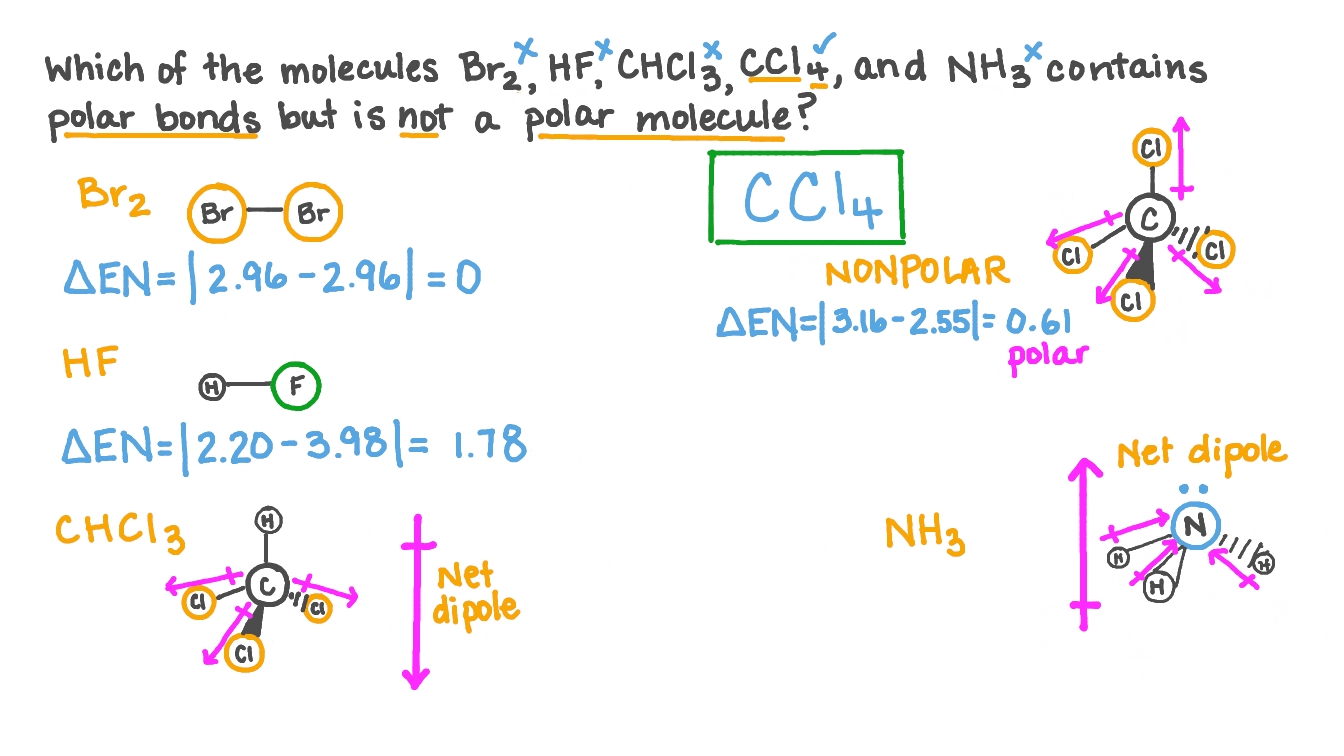
Question Video: Determining the Molecule That Contains Polar Bonds but Is Not a Polar Molecule | Nagwa

Carbon tetrachloride and water are immiscible whereas alcohol and water are miscible Explain on the basis of molecular structures - Chemistry - Solutions - 17010795 | Meritnation.com





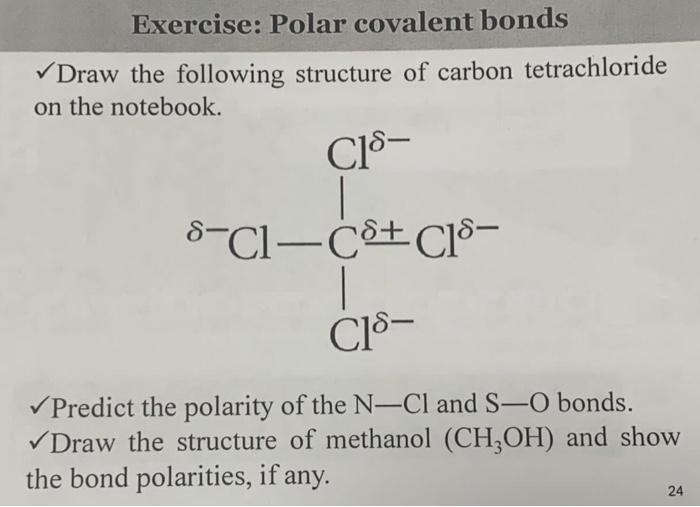

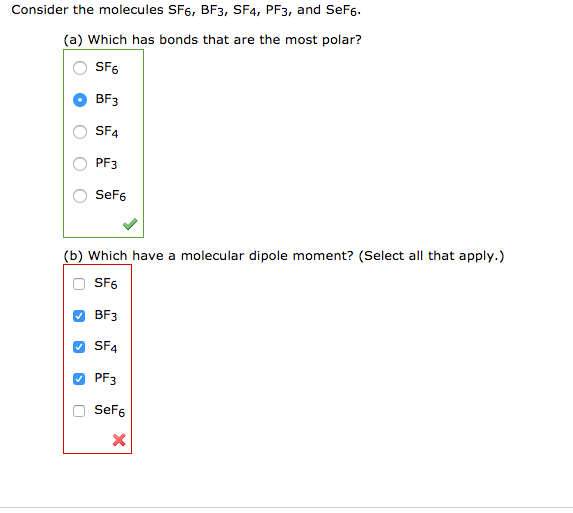
![Is \\[CC{l_4}\\] polar or nonpolar? Is \\[CC{l_4}\\] polar or nonpolar?](https://www.vedantu.com/question-sets/dbf3f5ee-35e7-43b7-b8a3-cfe63dfd520e2097468885289678636.png)




